In the highly regulated pharmaceutical industry, syringe labeling is not just a procedural task; it’s a critical component of product safety and regulatory compliance.
Anesthesia syringe labels play a critical role in preventing medication errors and ensuring patient safety. Ensuring that syringes are correctly labeled is crucial for patient safety, healthcare efficiency, and meeting the stringent standards set by global health authorities.
Importance of proper syringe labeling
- Regulatory compliance: Syringe labels must contain essential information such as drug name, dosage, manufacturer details, expiration date, and batch number. These details are mandatory to meet FDA and other international health regulations, helping to avoid legal issues and penalties.
- Patient safety: Labels provide vital information on the correct and safe use of the drug. Accurate labeling helps to prevent medical errors, ensuring that patients receive the correct medication. This is crucial in high-stakes environments such as anesthesiology, where clear labeling directly influences patient safety and adherence to proper dosing and drug identification. Accurate labeling also plays a critical role in ensuring medication safety, and preventing serious mistakes when syringes are mislabeled or unlabeled.
- Role of healthcare professionals: Whether medications are administered by a healthcare professional or the patient themselves, proper labeling of syringes is essential to prevent errors and enhance patient safety. Healthcare professionals play a critical role in ensuring the safety and correctness of medication delivery through proper labeling.
- Brand integrity: Consistent and precise labeling reinforces brand reliability and trustworthiness among consumers and healthcare providers.

Medication labeling content and design
- Mandatory Information: The label must display key information that includes, but is not limited to, drug concentration, volume, storage conditions, and usage instructions. Clear and standardized drug labels are crucial in reducing medication errors and enhancing patient safety, especially in high-pressure environments like anesthesiology. Additionally, labeling medication containers with specific information such as drug names, concentrations, and expiration dates is critical to enhance patient safety and minimize medication errors.
- Design Standards: Labels should be designed to remain intact under various storage conditions and during use. This means using materials that resist smudging, tearing, and fading.
Labeling techniques and technologies for anesthesia syringe labels
- Advanced labeling machines: Manufacturers should invest in high-quality labeling machinery that can handle precise application requirements, such as wrap-around or flag labeling techniques, which are common for syringes. Accurately labeling medication syringes is crucial to prevent serious medication errors, especially in the administration of injectable medications like vaccines and anesthesia. Viallabeller can provide you with reliable syringe labeling machines that fit your needs.
- Integration with production lines: Syringe labeling systems should be seamlessly integrated with existing pharmaceutical production lines to maintain efficiency and throughput without sacrificing accuracy. Clear labeling standards for pharmacy bulk packages are essential to reduce the risk of medication errors and enhance patient safety.
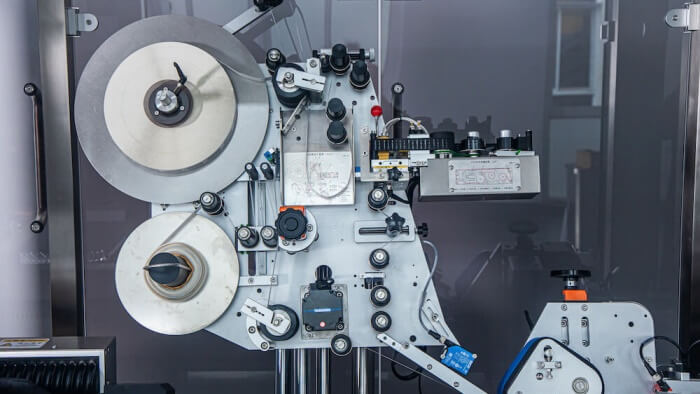
Quality control and compliance to prevent medication errors
- Regular audits: Implementing regular audits and checks throughout the labeling process ensures compliance with industry standards and helps identify areas for improvement. Providing information regarding potential drug interactions is crucial to ensure patient safety and inform healthcare professionals about the efficacy and risks associated with the medication.
- Use of technology: Employing advanced inspection systems like vision inspection technologies can help detect and reject syringes with missing or inaccurate labels, thus maintaining high standards of quality control. Prescription drug labeling plays a significant role in reducing medication use errors, particularly by improving labeling clarity and accessibility.
Challenges in syringe labeling
- High-speed production: Maintaining labeling accuracy in high-speed production environments is challenging and requires sophisticated equipment and well-trained personnel.
- Handling various syringe sizes: Adapting the labeling process to accommodate different sizes and types of syringes can complicate equipment setup and operation.
Conclusion
Effective syringe labeling is vital for ensuring that medical products are safe, compliant, and reliable. It is incumbent upon manufacturers to continually assess and upgrade their labeling processes to keep pace with technological advancements and regulatory changes. Emphasizing safe medication practices in labeling injectable medications is crucial to minimize harmful errors.
By prioritizing accurate labeling, manufacturers can significantly contribute to safeguarding public health while bolstering their market presence and reputation. Clear labeling of prescription drugs is essential to prevent medication errors and ensure patient safety, particularly for those with low health literacy or complex medication regimens. Implementing robust labeling systems and processes is not merely a regulatory obligation but a cornerstone of pharmaceutical best practices.







