Batch numbers are very important in making products, especially in pharmaceuticals. These numbers help track and ensure the quality and safety of items.
Manufacturing often happens in batches, not continuously. This helps control the process and keep things consistent. For medicines, it is very important to measure and record the ingredients in each batch.
This keeps a clear history and follows health rules. Knowing about batch numbers and their use is crucial for both makers and users.
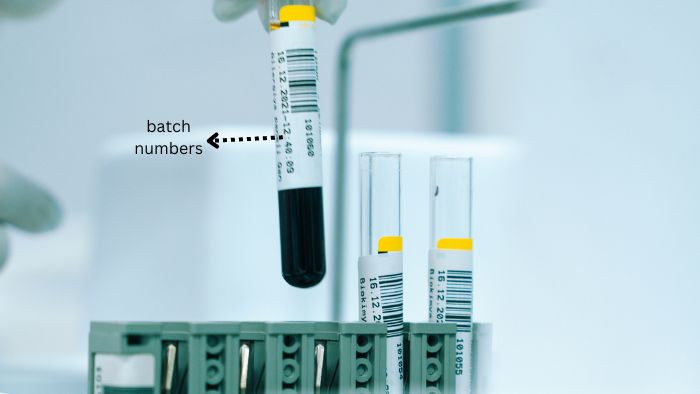
What are batch numbers?
Batch numbers are special codes given to groups of products made at the same time. These codes are very important for keeping track of products and making sure they are safe.
In the pharmaceutical industry, batch numbers help watch over production processes. It can manage stock, and follow rules.
If you think the text is too long, we have also carefully selected videos for you to answer:

Why use batch numbers
Batch numbers offer many important benefits. In this section, you can know why should use batch numbers.
Traceability
Batch numbers help track the history of products. This tracking is vital for solving problems in production or after distribution. If a product needs to be recalled, batch numbers help find the affected items quickly.
This reduces problems for consumers and allows quick fixes. Labels with batch numbers and expiry dates help keep track of products and follow rules.
Quality control
Keeping high standards of quality and safety is very important in the pharmaceutical industry. Batch numbers help check and verify product quality at each production stage.
Each batch gets a unique number for quality checks. This process helps prevent defects and makes sure products are safe and effective for consumers.
Inventory management
Pharma companies needs good inventory management. Batch numbers make tracking and managing inventory easier.
They help prevent running out of stock or having too much. This helps keep inventory levels right and reduces waste.

Viallabeller’s pharmaceutical labeling machines can label many containers. These include pill bottle labeling and other medicine packaging.
Regulatory requirements in the pharmaceutical industry
Pharmaceutical products must follow strict rules for safety and effectiveness. Batch numbers help meet these rules:
- Uniqueness: Each batch of medicine has a unique batch number. No numbers can repeat.
- Traceability: The batch number links to production and testing records.
- Clear to see: The batch number is on the package for easy reading.
- Coding rules: Different countries have rules for batch numbers. They often include the production date and serial number.
- Record keeping: Drug companies keep records of batch numbers for inspections.
Agencies like the FDA require batch numbers for tracking and checking quality. Following these rules helps keep people safe and supports the pharmaceutical industry.
Integration with modern technologies
Modern technology improves batch number tracking in drug manufacturing. Advanced software and machines make managing batch numbers easier. The following are the tools and methods:
- Barcode scanners: These devices read batch numbers on packages.
- Tracking software: Software helps organize and monitor batch numbers.
- RFID tags: These tags store batch numbers for easier tracking.
- Databases: Companies store batch numbers in digital databases.
- Labels: Batch numbers are printed on labels for easy identification.
These tools reduce mistakes and give real-time data. They also help with managing the entire production process.

Conclusion
Batch numbers help track and manage pharmaceutical products. They support traceability and quality control in production.
Batch numbers also help meet safety rules in the industry. Batch production improves efficiency and keeps products consistent. Batch numbers ensure that products stay safe and reliable.